Combustion is a process that occurs when a fuel reacts with an oxidizing agent, usually oxygen, to release energy in the form of heat and light. There are two types of combustion: ideal (upper) combustion and incomplete (lower) combustion. In this blog, we will discuss the differences between the two and their respective effects on the environment and human health.
Ideal (Upper) Combustion
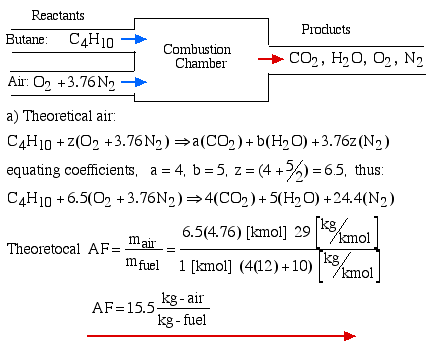
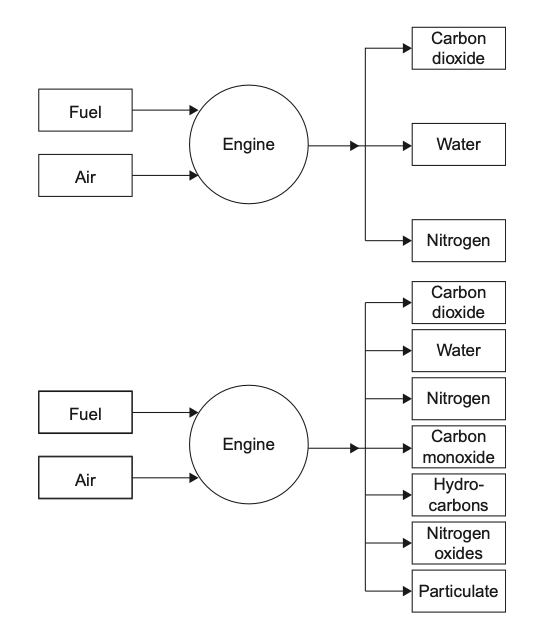
Ideal or upper combustion is a type of combustion in which a fuel is burned completely with sufficient oxygen. When a fuel undergoes ideal combustion, it reacts with oxygen to produce carbon dioxide and water vapor as the main by-products. The chemical equation for ideal combustion can be represented as:
Fuel + Oxygen → Carbon dioxide + Water Vapor + Heat Energy
For example, burning methane (CH4) completely with oxygen produces carbon dioxide (CO2) and water vapor (H2O), and releases heat energy:
CH4 + 2O2 → CO2 + 2H2O + heat energy
Ideal combustion is the most efficient and cleanest form of combustion. It produces the highest amount of heat energy per unit of fuel burned and releases no harmful pollutants or particulate matter into the air. It is commonly used in engines and power plants that require high efficiency and low emissions.
Incomplete (Lower) Combustion
Incomplete or lower combustion occurs when a fuel is burned with insufficient oxygen. This type of combustion results in the production of carbon monoxide, unburned hydrocarbons, and soot. The chemical equation for incomplete combustion can be represented as:
Fuel + Oxygen (insufficient) → Carbon Monoxide + Unburned Hydrocarbons + Soot + Heat Energy
For example, burning methane (CH4) incompletely with insufficient oxygen produces carbon monoxide (CO), unburned hydrocarbons (C2H2), soot, and releases heat energy:
CH4 + O2 (insufficient) → CO + C2H2 + Soot + heat energy
Incomplete combustion is less efficient and more polluting than ideal combustion. It produces less heat energy per unit of fuel burned and releases harmful pollutants and particulate matter into the air. It is commonly associated with the burning of wood, coal, and other solid fuels in traditional stoves, fireplaces, and furnaces.
Effects on the Environment and Human Health
Ideal combustion produces no harmful pollutants or particulate matter, and thus has minimal impact on the environment and human health. In contrast, incomplete combustion produces significant amounts of carbon monoxide, which is a poisonous gas that can cause headaches, dizziness, nausea, and even death. It also produces unburned hydrocarbons and soot, which can contribute to the formation of smog and air pollution.
Incomplete combustion also releases more greenhouse gases into the atmosphere than ideal combustion. Carbon dioxide, which is produced by both types of combustion, is a major contributor to climate change. However, incomplete combustion also produces carbon monoxide and other greenhouse gases such as methane, which are even more potent than carbon dioxide in trapping heat in the atmosphere.
Conclusion
Ideal (upper) and incomplete (lower) combustion are two different types of combustion that have significant differences in their efficiency, environmental impact, and effects on human health. Ideal combustion produces no harmful pollutants or particulate matter and is the most efficient and cleanest form of combustion. Incomplete combustion, on the other hand, produces harmful pollutants and particulate matter and is less efficient and more polluting than ideal combustion. It is important to understand the differences between the two types of combustion to minimize their impact on the environment and human health.
Ideal (Upper) Combustion
Ideal combustion occurs when a fuel is burned with the right amount of oxygen, allowing for complete combustion. Complete combustion means that all the fuel is burned and converted into carbon dioxide and water vapor. Ideal combustion is the most efficient way to burn fuel and produces the highest amount of heat energy per unit of fuel burned.
Ideal combustion is commonly used in engines, power plants, and other industrial applications where high efficiency and low emissions are required. For example, modern cars and trucks are equipped with catalytic converters that use ideal combustion to reduce harmful emissions such as carbon monoxide, nitrogen oxides, and hydrocarbons.
Incomplete (Lower) Combustion
Incomplete combustion occurs when a fuel is burned with insufficient oxygen, leading to the formation of carbon monoxide, unburned hydrocarbons, and soot. Incomplete combustion is less efficient than ideal combustion and produces less heat energy per unit of fuel burned.
Incomplete combustion is commonly associated with the burning of solid fuels such as wood, coal, and biomass in traditional stoves, fireplaces, and furnaces. These fuels are often burned in poorly ventilated spaces, leading to the accumulation of harmful pollutants and particulate matter in the air.
Effects on the Environment and Human Health
Ideal combustion produces minimal impact on the environment and human health because it produces no harmful pollutants or particulate matter. However, the carbon dioxide produced by ideal combustion is a major contributor to climate change.
Incomplete combustion, on the other hand, produces significant amounts of carbon monoxide, unburned hydrocarbons, and soot, which are harmful to human health and the environment. Carbon monoxide is a poisonous gas that can cause headaches, dizziness, nausea, and even death in high concentrations. Unburned hydrocarbons and soot can contribute to the formation of smog and air pollution, which can cause respiratory problems and other health issues.
Incomplete combustion also releases more greenhouse gases into the atmosphere than ideal combustion, including carbon monoxide, methane, and other pollutants. These gases contribute to climate change by trapping heat in the atmosphere and causing global warming.
Conclusion
In summary, ideal (upper) and incomplete (lower) combustion are two types of combustion that have different effects on the environment and human health. Ideal combustion is the most efficient and cleanest form of combustion, producing no harmful pollutants or particulate matter. Incomplete combustion, on the other hand, is less efficient and more polluting, producing harmful pollutants and particulate matter that can impact human health and the environment. It is important to understand the differences between the two types of combustion and their respective effects to minimize their impact on the environment and human health.
Advantages of Ideal (Upper) Combustion:
- High efficiency: Ideal combustion is the most efficient way to burn fuel, producing the highest amount of heat energy per unit of fuel burned.
- Low emissions: Ideal combustion produces minimal emissions of harmful pollutants and particulate matter, making it a cleaner burning process.
- Widely used: Ideal combustion is commonly used in engines, power plants, and other industrial applications where high efficiency and low emissions are required.
Disadvantages of Ideal (Upper) Combustion:
- Greenhouse gas emissions: Although ideal combustion produces minimal emissions of harmful pollutants and particulate matter, it still produces significant amounts of carbon dioxide, which is a major contributor to climate change.
- Dependence on fossil fuels: Ideal combustion typically requires the use of fossil fuels, which are finite resources that contribute to climate change.
Advantages of Incomplete (Lower) Combustion:
- Availability of fuels: Incomplete combustion can use a variety of fuels, including wood, coal, and biomass, which are widely available and renewable.
- Low cost: Incomplete combustion is often less expensive than ideal combustion because it requires less sophisticated technology.
- Heat output: Incomplete combustion can produce a higher heat output than ideal combustion because it does not burn all the fuel, leaving some unburned material that can generate additional heat.
Disadvantages of Incomplete (Lower) Combustion:
- Harmful emissions: Incomplete combustion produces significant amounts of harmful pollutants and particulate matter, which can negatively impact human health and the environment.
- Lower efficiency: Incomplete combustion is less efficient than ideal combustion, producing less heat energy per unit of fuel burned.
- Fire risk: Incomplete combustion can increase the risk of fires and explosions because of the buildup of unburned material and flammable gases.
It is important to note that both ideal (upper) and incomplete (lower) combustion have advantages and disadvantages depending on the specific situation and context in which they are used. It is important to consider the environmental and health impacts of both types of combustion and work to minimize their negative effects while maximizing their benefits.