Catalytic converters are an essential component of modern cars, designed to reduce the emissions of harmful pollutants from exhaust gases. They were first introduced in the 1970s in response to the increasing air pollution caused by the growing number of vehicles on the roads. Since then, they have become mandatory in most countries and have greatly contributed to reducing the levels of pollutants in the air.
What is a Catalytic Converter?
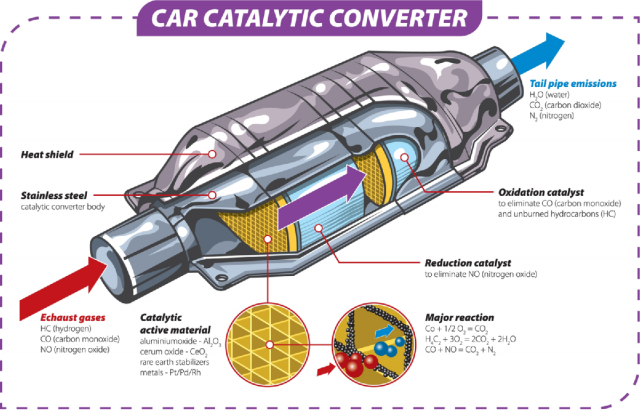
A catalytic converter is a device that is installed in a vehicle’s exhaust system to reduce the emissions of harmful pollutants. It works by converting harmful gases and pollutants, such as carbon monoxide, nitrogen oxides, and hydrocarbons, into less harmful gases, such as water vapor, carbon dioxide, and nitrogen. The device uses a catalyst, which is usually made of a combination of precious metals such as platinum, palladium, and rhodium, to facilitate the chemical reactions that occur within the converter.

How does it work?
The catalytic converter works by using a chemical reaction to convert harmful pollutants into less harmful gases. It does this through a process called catalysis, which involves the use of a catalyst to speed up a chemical reaction. When the exhaust gases pass through the converter, they come into contact with the catalyst, which causes the pollutants to break down into less harmful gases.
The process of catalysis involves a number of steps. First, the pollutants are absorbed onto the surface of the catalyst. Then, they react with oxygen in the air to form less harmful gases. For example, carbon monoxide reacts with oxygen to form carbon dioxide, and nitrogen oxides react with oxygen to form nitrogen and oxygen gas. Finally, the less harmful gases are released into the atmosphere.
Types of Catalytic Converters
There are two main types of catalytic converters: two-way and three-way. Two-way converters are designed to reduce the emissions of carbon monoxide and hydrocarbons. They do not have the ability to reduce nitrogen oxides. Three-way converters, on the other hand, are designed to reduce all three pollutants: carbon monoxide, hydrocarbons, and nitrogen oxides.
In addition to these two types, there are also several other types of catalytic converters, including diesel oxidation catalysts, lean NOx traps, and selective catalytic reduction systems. Each of these types is designed for specific applications and has its own set of advantages and disadvantages.
Maintenance and Replacement
Catalytic converters are designed to last for the life of the vehicle, but they can become damaged or worn out over time. Some of the most common causes of catalytic converter failure include damage from road debris, exposure to high temperatures, and exposure to certain chemicals.
When a catalytic converter fails, it can cause a number of problems, including reduced fuel efficiency, poor engine performance, and increased emissions. In most cases, the only solution is to replace the converter with a new one.
In conclusion, catalytic converters are an essential component of modern vehicles, designed to reduce the emissions of harmful pollutants from exhaust gases. They use a catalyst to facilitate chemical reactions that convert pollutants into less harmful gases. While they are designed to last for the life of the vehicle, they can become damaged or worn out over time and may need to be replaced. Regular maintenance can help ensure that the converter is working properly and can help extend its life.
History
The first catalytic converter was developed by a French engineer named Eugene Houdry in the 1940s. Houdry had previously worked on developing a process for extracting gasoline from oil shale, which produced large amounts of harmful pollutants. He realized that the same principles could be applied to reduce the emissions from cars, and he developed the first catalytic converter in the 1950s.
The technology was not widely used until the 1970s, when the US Environmental Protection Agency (EPA) introduced strict regulations on vehicle emissions. Since then, catalytic converters have become mandatory in most countries around the world.
Catalytic Converter Construction
A catalytic converter consists of a metal shell, a ceramic or metallic honeycomb substrate, and a catalyst. The substrate is coated with the catalyst, which is usually made of a combination of platinum, palladium, and rhodium.
The substrate is typically made of ceramic or metallic material and has a honeycomb-like structure. The honeycomb structure provides a large surface area for the exhaust gases to come into contact with the catalyst, which helps to increase the efficiency of the conversion process.
The metal shell is designed to protect the substrate and catalyst from damage and to provide a secure attachment to the exhaust system. The shell is usually made of stainless steel or other corrosion-resistant metals.
Catalytic Converter Efficiency
The efficiency of a catalytic converter depends on a number of factors, including the temperature of the exhaust gases, the ratio of air to fuel in the engine, and the quality of the catalyst.
For example, if the engine is running too rich (i.e., there is too much fuel in the air/fuel mixture), the converter may not be able to convert all of the pollutants in the exhaust gas. This can lead to increased emissions and reduced efficiency.
Similarly, if the converter is exposed to excessively high temperatures, it may become damaged or less efficient. This can happen if the engine is running too hot or if the converter is clogged or damaged.
Catalytic Converter Recycling
Catalytic converters contain precious metals such as platinum, palladium, and rhodium, which can be recycled and reused. Because these metals are expensive and in limited supply, there is a growing market for catalytic converter recycling.
The recycling process involves removing the catalyst from the substrate and refining it to recover the precious metals. The substrate can also be recycled, but it is typically less valuable than the catalyst.
Conclusion
Catalytic converters are a vital component of modern vehicles, helping to reduce the emissions of harmful pollutants and improve air quality. While they are designed to last for the life of the vehicle, they can become damaged or worn out over time and may need to be replaced. Regular maintenance and proper engine tuning can help ensure that the converter is working properly and can help extend its life. Additionally, catalytic converters contain valuable precious metals that can be recycled and reused, making them an important resource for the automotive industry.
Advantages:
- Reduced Emissions: The primary advantage of catalytic converters is that they reduce the amount of harmful pollutants that are released into the atmosphere. The three-way catalytic converter, which is the most common type of converter used in vehicles, can reduce emissions of carbon monoxide (CO), nitrogen oxides (NOx), and unburned hydrocarbons (HC).
- Improved Air Quality: By reducing emissions, catalytic converters help improve air quality and reduce the negative health effects associated with air pollution. This can help reduce the risk of respiratory problems and other health issues.
- Long Lifespan: Catalytic converters are designed to last for the life of the vehicle, and they require minimal maintenance. This means that once installed, drivers do not need to worry about replacing or maintaining the converter.
- Regulatory Compliance: Many countries and states have regulations in place that require vehicles to have a catalytic converter installed. By complying with these regulations, drivers can avoid fines and other penalties.
Disadvantages:
- Reduced Performance: One of the main disadvantages of catalytic converters is that they can reduce engine performance. This is because the converter restricts the flow of exhaust gases, which can reduce the engine’s power output.
- Expensive: Catalytic converters can be expensive to replace if they become damaged or worn out. This is because they contain precious metals such as platinum, palladium, and rhodium, which are expensive to mine and refine.
- Clogging: Catalytic converters can become clogged if they are exposed to excessive amounts of oil or other contaminants. This can reduce their effectiveness and cause engine performance problems.
- Temperature Sensitivity: Catalytic converters are sensitive to temperature changes, and they may not work properly if the engine is too hot or too cold. This can lead to increased emissions and reduced fuel efficiency.
Overall, while catalytic converters have some disadvantages, their advantages in reducing harmful emissions and improving air quality make them an important component of modern vehicles.