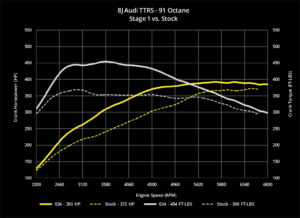
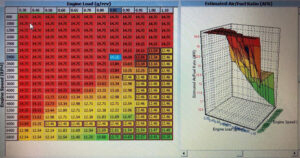
A good supply of electric power is necessary for modern vehicles. The engines require a large current to operate the starter motor, and many other systems are electrically powered. All modern cars use a 12V system. The majority of vehicle batteries are of conventional design, using lead plates in a dilute sulfuric acid electrolyte. This feature leads to the common description of ‘lead-acid’ batteries. The output from a lead-acid battery is direct current (d.c.). A rechargeable battery is an electrochemical unit that converts an electric current into a modified chemical compound. This chemical reaction can be reversed to release an electric current.
The modified chemical compound in the battery stores energy, which is available as electricity when connected to a circuit. A few batteries have open cells that require routine maintenance to the electrolyte level. This usually consists of adding distilled water at regular intervals. Most modern lead-acid battery designs have improved plate construction and case design, which with precise alternator charge control allows maintenance-free types to be used. A vehicle 12V battery is made up from six cells. Each lead-acid cell has a nominal voltage of 2.1V, which gives a value of 12.6V for a fully charged battery under no load conditions. The six cells are connected in series, internally in the battery, with lead bars. The cells are formed in the battery case and are completely separate from each other. Each cell has a set of interleaved positive and negative plates kept apart by porous separators. The separators prevent contact of the plates, which would give an internal short-circuit and affect the chemical reaction in the battery cell. The cell plates are supported above the bottom of the case. This leaves a sediment trap below the plates so that any loose material that falls to the bottom does not cause a short-circuit between the plates. The cell plates are formed in a lattice grid of lead–antimony or lead–calcium alloy. The grid carries the active material and acts as the electrical conductor. The active materials are lead peroxide for the positive plate and spongy lead for the negative plate. When a battery is in a charged state, the positive plates of lead peroxide (PbO2) are reddish brown, and the negative plates of spongy lead (Pb) are gray. When the battery is discharging, a chemical reaction with the electrolyte changes both plates to lead sulfate (PbSO4). Applying an electric current to the battery reverses the process. The charged battery stores chemical energy. This can be released as electrical energy when the battery is connected into a circuit. The electrolyte is dilute sulfuric acid, which reacts with the cell plate material during charging and discharging of the battery. Sulfuric acid (H2SO4) consists of hydrogen, sulfur and oxygen. These chemicals separate during the charge and discharge process and attach to the cell plate active material or return to the electrolyte. During discharge, the sulfate (SO4) combines with the lead to form lead sulfate (PbSO4). The oxygen in the positive plate is released to the electrolyte and combines with the hydrogen that is left, to form water (H2O). During charging, the reverse process occurs with the sulfate (SO4), leaving the cell plates to reform with the hydrogen in the electrolyte to produce sulfuric acid (H2SO4). Oxygen in the electrolyte is released to reform with the positive cell plate material as lead peroxide (PbO2).