Combustion is the process by which fuel is burned to release energy in the form of heat and light. This process is essential for many applications, including powering engines, generating electricity, and heating homes. Combustion occurs in several distinct phases, each with its unique characteristics and reactions. Understanding these phases is crucial for optimizing combustion processes and improving efficiency. In this blog, we will explore the different phases of combustion and their importance.
Phase 1: Initiation
The initiation phase is the first stage of combustion, during which a fuel source is heated to its ignition temperature. The ignition temperature is the minimum temperature at which a fuel will spontaneously ignite and start burning. Once the fuel reaches this temperature, it releases energy in the form of heat, which sustains the combustion process.
There are several ways to initiate combustion, including spark ignition, compression ignition, and chemical initiation. In spark ignition, a spark is used to ignite a fuel-air mixture. In compression ignition, the heat generated by compressing a fuel-air mixture raises the temperature above the ignition point. Chemical initiation involves using a catalyst or other chemical agent to trigger combustion.
Phase 2: Flame Propagation
Once combustion is initiated, the flame propagation phase begins. During this phase, the flame spreads through the fuel source, releasing energy in the form of heat and light. The speed at which the flame propagates depends on various factors, including fuel composition, temperature, pressure, and airflow.
Flame propagation can occur in several different ways, including deflagration, detonation, and slow combustion. Deflagration is a relatively slow flame propagation that occurs through a fuel-air mixture. Detonation is a much faster flame propagation that occurs when a high-pressure shockwave ignites the fuel source. Slow combustion is a more gradual process that occurs when a fuel source burns slowly over an extended period.
Phase 3: Burnout
The burnout phase is the final stage of combustion, during which the remaining fuel is consumed, and the flame extinguishes. This phase is critical for ensuring complete combustion and reducing emissions.
Incomplete combustion can result in the formation of harmful pollutants, such as carbon monoxide and nitrogen oxides. These pollutants can have significant environmental and health impacts, making burnout an essential phase of the combustion process.
During the burnout phase, the remaining fuel is consumed, and the temperature gradually decreases as the energy is released. Once the temperature falls below the ignition point, the flame extinguishes, and combustion ceases.
Conclusion
In conclusion, combustion is a complex process that occurs in several distinct phases, each with its unique characteristics and reactions. Understanding these phases is crucial for optimizing combustion processes, reducing emissions, and improving efficiency. By controlling the initiation, flame propagation, and burnout phases of combustion, we can harness the energy of fuel sources while minimizing their environmental impact.
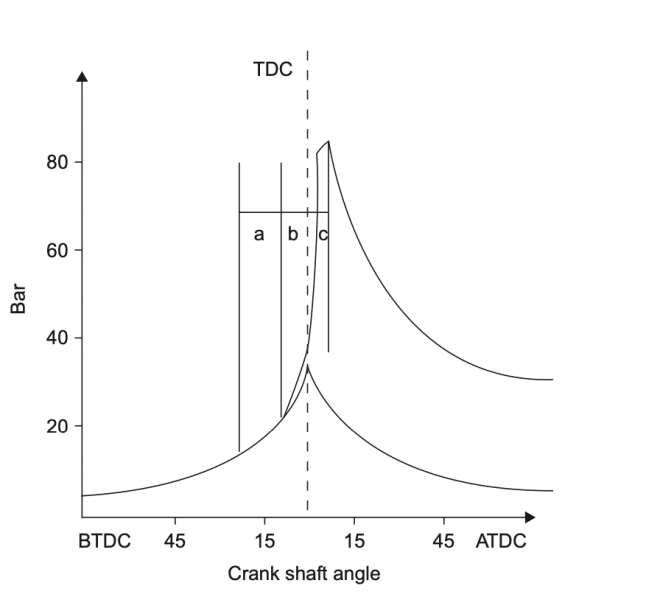
Phase 1: Initiation
The initiation phase of combustion involves heating the fuel source to its ignition temperature. This temperature varies depending on the fuel source and the conditions under which it is burned. For example, gasoline has an ignition temperature of around 495°F (257°C), while diesel fuel has an ignition temperature of around 450°F (232°C).
Once the fuel source reaches its ignition temperature, it releases energy in the form of heat. This heat sustains the combustion process by raising the temperature of the surrounding fuel and air mixture, which in turn causes more fuel molecules to break apart and react with oxygen. The reaction between the fuel and oxygen produces energy in the form of heat and light, which we perceive as flames.
There are several ways to initiate combustion, including spark ignition, compression ignition, and chemical initiation. Spark ignition is used in gasoline engines, where a spark plug ignites a fuel-air mixture in the combustion chamber. Compression ignition is used in diesel engines, where the heat generated by compressing a fuel-air mixture causes it to ignite spontaneously. Chemical initiation involves using a catalyst or other chemical agent to trigger combustion, such as in a catalytic converter in a car’s exhaust system.
Phase 2: Flame Propagation
Once combustion is initiated, the flame propagation phase begins. During this phase, the flame spreads through the fuel source, releasing energy in the form of heat and light. The speed at which the flame propagates depends on various factors, including fuel composition, temperature, pressure, and airflow.
Flame propagation can occur in several different ways, including deflagration, detonation, and slow combustion. Deflagration is a relatively slow flame propagation that occurs through a fuel-air mixture. It is the most common form of combustion in engines and other industrial processes. Detonation is a much faster flame propagation that occurs when a high-pressure shockwave ignites the fuel source. It is typically associated with explosive or high-performance applications, such as in rocket engines or internal combustion engines running at high speeds. Slow combustion is a more gradual process that occurs when a fuel source burns slowly over an extended period, such as in smoldering fires.
Phase 3: Burnout
The burnout phase is the final stage of combustion, during which the remaining fuel is consumed, and the flame extinguishes. This phase is critical for ensuring complete combustion and reducing emissions.
During the burnout phase, the remaining fuel is consumed, and the temperature gradually decreases as the energy is released. Once the temperature falls below the ignition point, the flame extinguishes, and combustion ceases. Incomplete combustion can result in the formation of harmful pollutants, such as carbon monoxide and nitrogen oxides. These pollutants can have significant environmental and health impacts, making burnout an essential phase of the combustion process.
In summary, the phases of combustion are initiation, flame propagation, and burnout. By understanding these phases and controlling the conditions under which they occur, we can optimize combustion processes, reduce emissions, and improve efficiency.
Advantages:
- High energy output: Combustion is a highly efficient process that releases a significant amount of energy in the form of heat and light. This energy can be harnessed to power engines, generate electricity, or heat buildings, among other applications.
- Wide range of fuel sources: Combustion can use a wide range of fuel sources, including fossil fuels like coal, oil, and natural gas, as well as renewable sources like biomass and biofuels. This versatility makes combustion a valuable tool for meeting energy needs in a variety of settings.
- Established technology: Combustion technology has been in use for centuries and is well-established in many industries, including power generation, transportation, and manufacturing. This means that combustion processes are often reliable and cost-effective.
- Provides heat and light: In addition to generating energy, combustion also produces heat and light, which can be used for heating, lighting, and other applications.
Disadvantages:
- Environmental impact: Combustion processes produce a variety of harmful pollutants, including carbon dioxide, nitrogen oxides, sulfur dioxide, particulate matter, and other greenhouse gases. These pollutants can contribute to climate change, air pollution, and other environmental problems.
- Health effects: The pollutants generated by combustion can have significant health impacts, particularly for vulnerable populations like children, the elderly, and those with respiratory or cardiovascular conditions.
- Limited fuel supply: Fossil fuels, which are the primary sources of fuel for many combustion processes, are a finite resource. As these resources are depleted, it will become increasingly challenging to meet energy needs through combustion processes.
- Fire hazard: Combustion processes can pose a fire hazard, particularly in industrial settings where combustible materials are present. Proper safety protocols and equipment must be in place to mitigate this risk.
In summary, combustion processes have several advantages and disadvantages. While combustion is a valuable tool for meeting energy needs, it is essential to consider its environmental and health impacts and work to mitigate them through improved technologies and practices.




